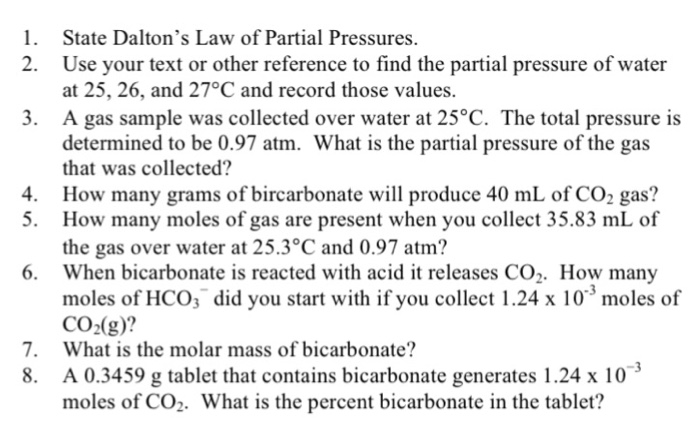
Mastering Dalton’s Law: A Comprehensive Guide to Partial Pressure
In the realm of chemistry, understanding the behavior of gases is paramount. Among the fundamental principles governing gas behavior, Dalton’s Law of Partial Pressures stands out as a cornerstone. This law, formulated by the renowned chemist John Dalton, provides a crucial framework for comprehending the pressure exerted by a mixture of gases. For students and professionals alike, grappling with this concept often involves working through exercises. This guide delves into the practical application of Dalton’s Law, focusing on the use of worksheets and providing insights to help you master the subject.
This article will explore the core principles of Dalton’s Law of Partial Pressures, illustrate how to work through problems using worksheets, and provide access to resources, including PDF versions of Dalton’s Law of Partial Pressure worksheets with answers. Whether you’re a student preparing for an exam or a professional seeking to reinforce your knowledge, this guide is designed to offer clarity and practical assistance.
Understanding Dalton’s Law of Partial Pressures
Dalton’s Law of Partial Pressures, in essence, states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas component. The partial pressure of a gas is the pressure that the gas would exert if it occupied the same volume alone. This law is predicated on the assumption that the gases in the mixture do not react with each other and that they behave ideally.
Mathematically, Dalton’s Law is expressed as:
Ptotal = P1 + P2 + P3 + … + Pn
Where:
- Ptotal is the total pressure of the gas mixture
- P1, P2, P3, …, Pn are the partial pressures of each gas component
For example, if you have a mixture of oxygen, nitrogen, and carbon dioxide, the total pressure of the mixture would be the sum of the partial pressure of oxygen, the partial pressure of nitrogen, and the partial pressure of carbon dioxide.
The Significance of Dalton’s Law
Dalton’s Law of Partial Pressures is not merely an academic concept; it has significant applications across various fields. In industrial processes, understanding the behavior of gas mixtures is critical for designing and optimizing chemical reactions. In the field of environmental science, it helps in analyzing atmospheric composition and understanding pollution. Furthermore, in the medical field, it is crucial for understanding respiration and the delivery of oxygen to the body.
For instance, divers use gas mixtures, and understanding the partial pressures of the gases (oxygen, nitrogen, helium) is vital for their safety. Similarly, in the study of air quality, measuring the partial pressures of pollutants is essential for assessing environmental hazards.
Working with Dalton’s Law of Partial Pressure Worksheets
To truly grasp Dalton’s Law, practice is essential. This is where worksheets come into play. A Dalton’s Law of Partial Pressure worksheet typically presents a series of problems that require you to apply the law to calculate either the total pressure of a gas mixture or the partial pressure of a specific gas component. The problems often involve scenarios with known volumes, temperatures, and amounts of gases.
The first step in solving a Dalton’s Law of Partial Pressure worksheet problem is to identify the knowns and the unknowns. Clearly state what information is provided in the problem and what you are trying to find. Next, determine the appropriate formula to use. In most cases, you will use the equation for Dalton’s Law. Some problems may also require you to use the ideal gas law (PV = nRT) to calculate the number of moles or the volume of a gas.
Once you have determined the formula, plug in the known values and solve for the unknown. Be sure to pay attention to the units of measurement and convert them if necessary to ensure consistency. Finally, double-check your answer to make sure it makes sense in the context of the problem. Does the calculated total pressure seem reasonable given the partial pressures of the individual gases? Are the units correct?
A Dalton’s Law of Partial Pressure worksheet with answers is a valuable tool for self-assessment. By comparing your solutions to the provided answers, you can identify areas where you need to improve. This allows you to focus your study efforts on the concepts that are most challenging for you.
Common Types of Problems in Dalton’s Law Worksheets
Dalton’s Law of Partial Pressure worksheets typically include a variety of problem types to test your understanding of the concept. Here are some common examples:
- Calculating Total Pressure: Given the partial pressures of the individual gases in a mixture, calculate the total pressure.
- Calculating Partial Pressure: Given the total pressure and the partial pressures of some of the gases, calculate the partial pressure of the remaining gas.
- Using the Ideal Gas Law: Problems that require you to use the ideal gas law (PV = nRT) in conjunction with Dalton’s Law to calculate the number of moles, volume, or other properties of the gases.
- Mixtures of Gases: Problems that involve mixtures of gases with different molar masses and properties.
- Applications in Real-World Scenarios: Problems that relate to real-world applications of Dalton’s Law, such as the composition of air or the behavior of gases in industrial processes.
Finding and Utilizing Dalton’s Law of Partial Pressure Worksheets with Answers (PDF)
The internet is a treasure trove of resources for chemistry students and enthusiasts. You can find numerous Dalton’s Law of Partial Pressure worksheets with answers in PDF format by conducting a simple search. Look for reputable educational websites, online learning platforms, and chemistry textbooks. Many educational institutions also provide free access to worksheets and answer keys on their websites.
When downloading Dalton’s Law of Partial Pressure worksheets with answers PDF, ensure the source is reliable and the content is accurate. A well-structured worksheet will include a variety of problems, ranging in difficulty, to challenge your understanding. The answer key should be clear, concise, and provide step-by-step solutions to help you understand how to arrive at the correct answer.
Once you have downloaded the worksheet, take time to work through the problems independently. Try to solve each problem without looking at the answer key. This will help you identify your strengths and weaknesses. After completing the problems, check your answers against the answer key. If you made any mistakes, review the solution and try to understand where you went wrong.
The availability of a Dalton’s Law of Partial Pressure worksheet with answers PDF significantly enhances the learning experience. It offers immediate feedback, allowing you to correct errors and reinforce your understanding of the concepts. By consistently practicing and reviewing your work, you can build confidence and master Dalton’s Law of Partial Pressures.
Tips for Success with Dalton’s Law Worksheets
To maximize your success when working with Dalton’s Law of Partial Pressure worksheets, consider the following tips:
- Review the Basics: Before starting the worksheets, make sure you have a solid understanding of the ideal gas law, molar mass, and the concept of partial pressure.
- Understand the Units: Pay close attention to the units of measurement (e.g., Pascals, atmospheres, mmHg) and make sure you convert them appropriately if necessary.
- Practice Regularly: The more you practice, the better you will become at solving problems related to Dalton’s Law.
- Seek Help When Needed: Don’t hesitate to ask your teacher, professor, or classmates for help if you are struggling with a particular concept.
- Work Through Examples: Before attempting the worksheets, work through the example problems provided in your textbook or online resources.
- Use a Calculator: A scientific calculator can be invaluable for solving complex problems and performing calculations quickly and accurately.
- Check Your Work: Always double-check your answers to ensure they are correct.
Beyond the Worksheet: Further Study and Resources
While Dalton’s Law of Partial Pressure worksheets with answers are a great starting point, it is important to supplement your learning with other resources. Consider the following:
- Textbooks: Consult your chemistry textbook for a detailed explanation of Dalton’s Law and related concepts.
- Online Tutorials: Many websites and YouTube channels offer video tutorials that explain Dalton’s Law in a clear and concise manner.
- Practice Quizzes: Take online quizzes to test your understanding and identify areas where you need to improve.
- Study Groups: Collaborate with your classmates to discuss concepts and solve problems together.
By combining the use of Dalton’s Law of Partial Pressure worksheets with other study resources, you can develop a comprehensive understanding of this important concept and excel in your chemistry studies.
Conclusion
Dalton’s Law of Partial Pressures is a fundamental principle in chemistry with wide-ranging applications. By working through Dalton’s Law of Partial Pressure worksheets with answers, you can solidify your understanding of this law and its practical implications. Remember to practice regularly, seek help when needed, and supplement your learning with other resources. With dedication and effort, you can master Dalton’s Law and excel in your chemistry studies. The ability to effectively utilize and interpret information from a Dalton’s Law of Partial Pressure worksheet will be an invaluable skill as you progress in your scientific endeavors.
For further exploration, consider researching the kinetic molecular theory of gases, which provides the theoretical underpinnings for Dalton’s Law. [See also: Kinetic Molecular Theory Explained] Also, investigate how Dalton’s Law relates to real-world phenomena such as gas diffusion and the behavior of gases in confined spaces. [See also: Gas Diffusion and Its Applications]

